Which Statement Describes An Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the move of those ions, but not conducting electrons.[1] [two] [3] This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes as well exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.[4] [5]
Electrically, such a solution is neutral. If an electrical potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are fatigued to the electrode that has a deficit of electrons. The motility of anions and cations in opposite directions within the solution amounts to a current. Some gases, such equally hydrogen chloride (HCl), under weather of high temperature or low pressure can besides role as electrolytes.[ clarification needed ] Electrolyte solutions tin can also event from the dissolution of some biological (east.thousand., Deoxyribonucleic acid, polypeptides) or synthetic polymers (e.thou., polystyrene sulfonate), termed "polyelectrolytes", which contain charged functional groups. A substance that dissociates into ions in solution or in the melt acquires the capacity to carry electricity. Sodium, potassium, chloride, calcium, magnesium, and phosphate in a liquid phase are examples of electrolytes.
In medicine, electrolyte replacement is needed when a person has prolonged vomiting or diarrhea, and as a response to sweating due to strenuous athletic activity. Commercial electrolyte solutions are available, especially for sick children (such as oral rehydration solution, Suero Oral, or Pedialyte) and athletes (sports drinks). Electrolyte monitoring is important in the treatment of anorexia and bulimia.
In science, electrolytes are one of the main components of electrochemical cells.[two]
Etymology [edit]
The discussion electrolyte derives from Aboriginal Greek ήλεκτρο- (ēlectro-), prefix related to electricity, and λυτός (lytos), pregnant "able to be untied or loosened".[6]
History [edit]
Svante Arrhenius, father of the concept of electrolyte dissociation in aqueous solution for which he received the Nobel Prize in Chemical science in 1903.
In his 1884 dissertation, Svante Arrhenius put along his caption of solid crystalline salts disassociating into paired charged particles when dissolved, for which he won the 1903 Nobel Prize in Chemistry.[seven] [8] [nine] [ten] Arrhenius's explanation was that in forming a solution, the common salt dissociates into charged particles, to which Michael Faraday (1791-1867) had given the name "ions" many years earlier. Faraday's belief had been that ions were produced in the procedure of electrolysis. Arrhenius proposed that, even in the absenteeism of an electric current, solutions of salts independent ions. He thus proposed that chemic reactions in solution were reactions between ions.[8] [9] [x]
Shortly later Arrhenius's hypothesis of ions, Franz Hofmeister and Siegmund Lewith[11] [12] [thirteen] found that different ion types displayed different effects on such things equally the solubility of proteins. A consistent ordering of these dissimilar ions on the magnitude of their effect arises consistently in many other systems as well. This has since become known as the Hofmeister series. While the origins of these effects are not abundantly clear and have been debated throughout the past century, it has been suggested that the charge density of these ions is important[fourteen] and might actually have explanations originating from the work of Charles-Augustin de Coulomb over 200 years ago.
Formation [edit]
Electrolyte solutions are normally formed when salt is placed into a solvent such as h2o and the individual components dissociate due to the thermodynamic interactions between solvent and solute molecules, in a process chosen "solvation". For example, when table salt (sodium chloride), NaCl, is placed in h2o, the salt (a solid) dissolves into its component ions, according to the dissociation reaction[ citation needed ]
- NaCl(s) → Na+ (aq) + Cl− (aq)
Information technology is besides possible for substances to react with h2o, producing ions. For example, carbon dioxide gas dissolves in water to produce a solution that contains hydronium, carbonate, and hydrogen carbonate ions.[ citation needed ]
Molten salts can besides be electrolytes as, for example, when sodium chloride is molten, the liquid conducts electricity. In item, ionic liquids, which are molten salts with melting points below 100 °C,[15] are a type of highly conductive non-aqueous electrolytes and thus accept constitute more than and more than applications in fuel cells and batteries.[xvi]
An electrolyte in a solution may exist described equally "full-bodied" if it has a high concentration of ions, or "dilute" if it has a low concentration. If a high proportion of the solute dissociates to form free ions, the electrolyte is stiff; if nigh of the solute does non dissociate, the electrolyte is weak. The properties of electrolytes may be exploited using electrolysis to extract elective elements and compounds contained within the solution.[ citation needed ]
Alkaline earth metals form hydroxides that are stiff electrolytes with limited solubility in water, due to the strong attraction between their constituent ions. This limits their awarding to situations where high solubility is required.[17]
In 2021 researchers accept institute that electrolyte can "substantially facilitate electrochemical corrosion studies in less conductive media".[xviii]
Physiological importance [edit]
In physiology, the chief ions of electrolytes are sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), hydrogen phosphate (HPO4 2−), and hydrogen carbonate (HCOiii −).[19] [ failed verification ] The electric charge symbols of plus (+) and minus (−) betoken that the substance is ionic in nature and has an imbalanced distribution of electrons, the result of chemical dissociation. Sodium is the principal electrolyte found in extracellular fluid and potassium is the principal intracellular electrolyte;[xx] both are involved in fluid residual and blood pressure level control.[21]
All known multicellular lifeforms crave a subtle and complex electrolyte residuum betwixt the intracellular and extracellular environments.[nineteen] In particular, the maintenance of precise osmotic gradients of electrolytes is important. Such gradients affect and regulate the hydration of the torso as well as blood pH, and are critical for nervus and muscle office. Various mechanisms exist in living species that keep the concentrations of dissimilar electrolytes under tight control.[ citation needed ]
Both muscle tissue and neurons are considered electric tissues of the body. Muscles and neurons are activated by electrolyte activity betwixt the extracellular fluid or interstitial fluid, and intracellular fluid. Electrolytes may enter or leave the cell membrane through specialized poly peptide structures embedded in the plasma membrane chosen "ion channels". For case, muscle wrinkle is dependent upon the presence of calcium (Ca2+), sodium (Na+), and potassium (One thousand+). Without sufficient levels of these key electrolytes, musculus weakness or severe muscle contractions may occur.[ citation needed ]
Electrolyte residual is maintained by oral, or in emergencies, intravenous (Iv) intake of electrolyte-containing substances, and is regulated by hormones, in general with the kidneys flushing out backlog levels. In humans, electrolyte homeostasis is regulated by hormones such as antidiuretic hormones, aldosterone and parathyroid hormones. Serious electrolyte disturbances, such as dehydration and overhydration, may lead to cardiac and neurological complications and, unless they are speedily resolved, will consequence in a medical emergency.
Measurement [edit]
Measurement of electrolytes is a ordinarily performed diagnostic procedure, performed via blood testing with ion-selective electrodes or urinalysis by medical technologists. The interpretation of these values is somewhat meaningless without analysis of the clinical history and is often impossible without parallel measurements of renal function. The electrolytes measured most oftentimes are sodium and potassium. Chloride levels are rarely measured except for arterial blood gas interpretations since they are inherently linked to sodium levels. I important test conducted on urine is the specific gravity test to determine the occurrence of an electrolyte imbalance.[ citation needed ]
Rehydration [edit]
In oral rehydration therapy, electrolyte drinks containing sodium and potassium salts replenish the body's water and electrolyte concentrations afterwards dehydration caused by do, excessive booze consumption, diaphoresis (heavy sweating), diarrhea, vomiting, intoxication or starvation. Athletes exercising in extreme conditions (for iii or more than hours continuously, e.g. a marathon or triathlon) who do non swallow electrolytes risk dehydration (or hyponatremia).[22]
A home-made electrolyte potable can be made by using water, carbohydrate and salt in precise proportions.[23] It is of import to include glucose (carbohydrate) to utilise the co-ship mechanism of sodium and glucose. Commercial preparations are too available[24] for both human being and veterinarian apply.
Electrolytes are commonly found in fruit juices, sports drinks, milk, nuts, and many fruits and vegetables (whole or in juice form) (e.g., potatoes, avocados).
Electrochemistry [edit]
When electrodes are placed in an electrolyte and a voltage is applied, the electrolyte will conduct electricity. Solitary electrons normally cannot pass through the electrolyte; instead, a chemical reaction occurs at the cathode, providing electrons to the electrolyte. Some other reaction occurs at the anode, consuming electrons from the electrolyte. As a result, a negative accuse cloud develops in the electrolyte around the cathode, and a positive charge develops effectually the anode. The ions in the electrolyte neutralize these charges, enabling the electrons to keep flowing and the reactions to continue.[ citation needed ]
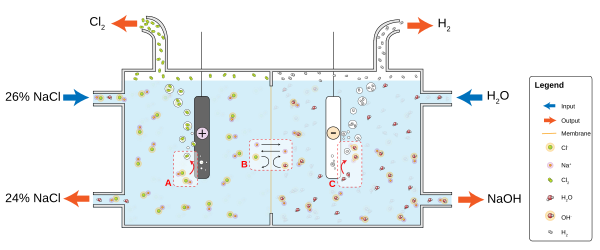
For example, in a solution of ordinary table table salt (sodium chloride, NaCl) in water, the cathode reaction volition be
- two H2O + 2e− → two OH− + Htwo
and hydrogen gas will bubble up; the anode reaction is
- two NaCl → 2 Na+ + Cl2 + 2e−
and chlorine gas will be liberated into solution where it reacts with the sodium and hydroxyl ions to produce sodium hypochlorite - household bleach. The positively charged sodium ions Na+ will react toward the cathode, neutralizing the negative charge of OH− in that location, and the negatively charged hydroxide ions OH− will react toward the anode, neutralizing the positive accuse of Na+ there. Without the ions from the electrolyte, the charges around the electrode would wearisome down continued electron menses; diffusion of H+ and OH− through h2o to the other electrode takes longer than movement of the much more prevalent table salt ions. Electrolytes dissociate in water considering h2o molecules are dipoles and the dipoles orient in an energetically favorable manner to solvate the ions.
In other systems, the electrode reactions can involve the metals of the electrodes as well as the ions of the electrolyte.
Electrolytic conductors are used in electronic devices where the chemical reaction at a metallic-electrolyte interface yields useful furnishings.
- In batteries, 2 materials with dissimilar electron affinities are used every bit electrodes; electrons flow from 1 electrode to the other outside of the battery, while inside the bombardment the excursion is airtight by the electrolyte's ions. Here, the electrode reactions convert chemic energy to electrical free energy.[25]
- In some fuel cells, a solid electrolyte or proton conductor connects the plates electrically while keeping the hydrogen and oxygen fuel gases separated.[26]
- In electroplating tanks, the electrolyte simultaneously deposits metal onto the object to exist plated, and electrically connects that object in the circuit.
- In operation-hours gauges, 2 thin columns of mercury are separated by a small electrolyte-filled gap, and, as charge is passed through the device, the metal dissolves on i side and plates out on the other, causing the visible gap to slowly move along.
- In electrolytic capacitors the chemical effect is used to produce an extremely thin dielectric or insulating coating, while the electrolyte layer behaves as one capacitor plate.
- In some hygrometers the humidity of air is sensed by measuring the conductivity of a nearly dry electrolyte.
- Hot, softened drinking glass is an electrolytic conductor, and some glass manufacturers go on the glass molten by passing a large electric current through it.
Solid electrolytes [edit]
Solid electrolytes can exist generally divided into 4 groups described beneath.
Gel electrolytes [edit]
Gel electrolytes – closely resemble liquid electrolytes. In essence, they are liquids in a flexible lattice framework. Various additives are ofttimes applied to increase the conductivity of such systems.[25] [27]
Polymer electrolytes [edit]
Dry polymer electrolytes – differ from liquid and gel electrolytes in the sense that common salt is dissolved directly into the solid medium. Usually it is a relatively high dielectric constant polymer (PEO, PMMA, PAN, polyphosphazenes, siloxanes, etc.) and a common salt with low lattice free energy. In society to increase the mechanical force and conductivity of such electrolytes, very often composites are used, and inert ceramic phase is introduced. There are ii major classes of such electrolytes: polymer-in-ceramic, and ceramic-in-polymer.[28] [29] [thirty]
Ceramic electrolytes [edit]
Solid ceramic electrolytes – ions migrate through the ceramic phase by means of vacancies or interstitials inside the lattice. In that location are besides glassy-ceramic electrolytes.
Organic plastic electrolytes [edit]
Organic ionic plastic crystals – are a type organic salts exhibiting mesophases (i.e. a state of affair intermediate between liquid and solid), in which mobile ions are orientationally or rotationally disordered while their centers are located at the ordered sites in the crystal structure.[26] They take various forms of disorder due to one or more solid–solid phase transitions below the melting betoken and have therefore plastic backdrop and good mechanical flexibility equally well every bit improved electrode|electrolyte interfacial contact. In particular, protic organic ionic plastic crystals (POIPCs),[26] which are solid protic organic salts formed by proton transfer from a Brønsted acid to a Brønsted base of operations and in essence are protic ionic liquids in the molten state, have plant to exist promising solid-state proton conductors for fuel cells. Examples include i,2,4-triazolium perfluorobutanesulfonate[26] and imidazolium methanesulfonate.[31]
Come across also [edit]
- Potent electrolyte
- Common salt span
- ITIES (interface between two immiscible electrolyte solutions)
- Ion transport number
- Elektrolytdatenbank Regensburg
- VTPR
- Electrochemical machining
References [edit]
- ^ Enderby, J Due east; Neilson, G W (1 June 1981). "The construction of electrolyte solutions". Reports on Progress in Physics. 44 (6): 593–653. doi:10.1088/0034-4885/44/6/001. ISSN 0034-4885. S2CID 250852242.
- ^ a b Petrovic, Slobodan (29 October 2020). Battery engineering crash course : a concise introduction. ISBN978-3-030-57269-3. OCLC 1202758685.
- ^ Winie, Tan; Arof, Abdul K.; Thomas, Sabu (xviii Feb 2020). Polymer Electrolytes: Label Techniques and Energy Applications. John Wiley & Sons. ISBN978-3-527-34200-6.
- ^ Wilkins, Lippincott Williams & (2007). Fluids and Electrolytes. Lippincott Williams & Wilkins. ISBN978-i-58255-923-0.
- ^ "electrolyte". National Cancer Institute. 2 February 2011. Archived from the original on 23 April 2018. Retrieved 18 December 2021.
- ^ "Electrolyte - Definition, List of Electrolytes and Examples with Videos". BYJUS . Retrieved 10 July 2022.
- ^ "The Nobel Prize in Chemistry 1903". Retrieved 5 January 2017.
- ^ a b Harris, William; Levey, Judith, eds. (1975). The New Columbia Encyclopedia (4th ed.). New York City: Columbia University. p. 155. ISBN978-0-231035-729.
- ^ a b McHenry, Charles, ed. (1992). The New Encyclopædia Britannica. Vol. i (15 ed.). Chicago: Encyclopædia Britannica, Inc. p. 587. Bibcode:1991neb..book.....Grand. ISBN978-085-229553-3.
- ^ a b Cillispie, Charles, ed. (1970). Dictionary of Scientific Biography (ane ed.). New York Urban center: Charles Scribner'south Sons. pp. 296–302. ISBN978-0-684101-125.
- ^ Franz Hofmeister (1888). "Zur Lehre Von Der Wirkung Der Salze". Naunyn-Schmiedeberg's Curvation. Pharmacol.
- ^ West. Kunz; J. Henle; B. W. Ninham (2004). "'Zur Lehre von der Wirkung der Salze' (about the science of the effect of salts): Franz Hofmeister's historical papers". Current Opinion in Colloid & Interface Science. 9 (1–2): 19–37. doi:10.1016/j.cocis.2004.05.005.
- ^ Gregory, Kasimir P.; Elliott, Gareth R.; Robertson, Hayden; Kumar, Anand; Wanless, Erica J.; Webber, Grant B.; Craig, Vincent S. J.; Andersson, Gunther G.; Page, Alister J. (2022). "Understanding specific ion effects and the Hofmeister series". Physical Chemistry Chemical Physics. 24 (21): 12682–12718. doi:10.1039/D2CP00847E. PMID 35543205.
- ^ Kasimir P. Gregory; Erica J. Wanless; Grant B. Webber; Vince S. J. Craig; Alister J. Page (2021). "The Electrostatic Origins of Specific Ion Effects: Quantifying the Hofmeister Serial for Anions". Chem. Sci. 12 (45): 15007–15015. doi:10.1039/D1SC03568A. PMC8612401. PMID 34976339. S2CID 244578563.
- ^ Shi, Jiahua (石家华); Sun, Xun (孙逊); Chunhe (杨春和), Yang; Gao, Qingyu (高青雨); Li, Yongfang (李永舫) (2002). 离子液体研究进展 (PDF). 化学通报 (in Simplified Chinese) (4): 243. ISSN 0441-3776. Archived from the original (PDF) on ii March 2017. Retrieved 1 March 2017.
- ^ Jiangshui Luo; Jin Hu; Wolfgang Saak; Rüdiger Beckhaus; Gunther Wittstock; Ivo F. J. Vankelecom; Carsten Agert; Olaf Conrad (2011). "Protic ionic liquid and ionic melts prepared from methanesulfonic acid and 1H-1,2,four-triazole as high temperature PEMFC electrolytes". Journal of Materials Chemistry. 21 (28): 10426–10436. doi:10.1039/C0JM04306K. S2CID 94400312.
- ^ Brown, Chemistry: The Key Science, 14th edition, pg. 680.
- ^ Matějovský, Lukáš; Staš, Martin; Dumská, Karolina; Pospíšil, Milan; Macák, Jan (one January 2021). "Electrochemical corrosion tests in an surround of low-conductive ethanol-gasoline blends: Function 1 – Testing of supporting electrolytes". Journal of Electroanalytical Chemical science. 880: 114879. doi:10.1016/j.jelechem.2020.114879. ISSN 1572-6657. S2CID 229508133.
- ^ a b Alfarouk, Khalid O.; Ahmed, Samrein B. Thousand.; Ahmed, Ahmed; Elliott, Robert L.; Ibrahim, Muntaser E.; Ali, Heyam S.; Wales, Christian C.; Nourwali, Ibrahim; Aljarbou, Ahmed N.; Bashir, Adil H. H.; Alhoufie, Sari T. South.; Alqahtani, Saad Saeed; Cardone, Rosa A.; Fais, Stefano; Harguindey, Salvador; Reshkin, Stephan J. (7 April 2020). "The Coaction of Dysregulated pH and Electrolyte Imbalance in Cancer". Cancers. 12 (iv): 898. doi:10.3390/cancers12040898. PMC7226178. PMID 32272658.
- ^ Ye, Shenglong (叶胜龙); Tang, Zhaoyou (汤钊猷) (1986). 细胞膜钠泵及其临床意义. 上海医学 [Shanghai Medicine] (in Simplified Chinese) (ane): 1.
- ^ Tu, Zhiquan (涂志全) (2004). 张定昌. 电解质紊乱对晚期肿瘤的治疗影响. 中华中西医杂志 [Chinese Mag of Chinese and Western Medicine] (in Simplified Chinese) (10).
在正常人体内,钠离子占细胞外液阳离子总量的92%,钾离子占细胞内液阳离子总量的98%左右。钠、钾离子的相对平衡,维持着整个细胞的功能和结构的完整。钠、钾是人体内最主要的电解质成分...
- ^ J, Estevez E; Baquero Eastward; Mora-Rodriguez R (2008). "Anaerobic functioning when rehydrating with water or commercially available sports drinks during prolonged practise in the heat". Applied Physiology, Nutrition, and Metabolism. 33 (2): 290–298. doi:x.1139/H07-188. PMID 18347684.
- ^ "Rehydration drinks". Webmd.com. 28 April 2008. Archived from the original on 23 October 2008. Retrieved 25 Dec 2018.
- ^ "Oral Rehydration Salt Suppliers". Rehydrate.org. 7 October 2014. Retrieved four December 2014.
- ^ a b Kamil Perzyna; Regina Borkowska; Jaroslaw Syzdek; Aldona Zalewska; Wladyslaw Wieczorek (2011). "The effect of additive of Lewis acid type on lithium–gel electrolyte characteristics". Electrochimica Acta. 57: 58–65. doi:10.1016/j.electacta.2011.06.014.
- ^ a b c d Jiangshui Luo; Annemette H. Jensen; Neil R. Brooks; Jeroen Sniekers; Martin Knipper; David Aili; Qingfeng Li; Bram Vanroy; Michael Wübbenhorst; Feng Yan; Luc Van Meervelt; Zhigang Shao; Jianhua Fang; Zheng-Hong Luo; Dirk Due east. De Vos; Koen Binnemans; Jan Fransaer (2015). "1,2,4-Triazolium perfluorobutanesulfonate every bit an archetypal pure protic organic ionic plastic crystal electrolyte for all-solid-state fuel cells". Energy & Environmental Science. 8 (4): 1276–1291. doi:10.1039/C4EE02280G. S2CID 84176511.
- ^ "The Roll-to-Curlicue Bombardment Revolution". Ev World. Archived from the original on 10 July 2011. Retrieved twenty August 2010.
- ^ Syzdek J, Borkowska R, Perzyna Grand, Tarascon JM, Wieczorek W (2007). "Novel composite polymeric electrolytes with surface-modified inorganic fillers". Journal of Power Sources. 173 (2): 712–720. Bibcode:2007JPS...173..712S. doi:10.1016/j.jpowsour.2007.05.061. ISSN 0378-7753.
- ^ Syzdek J, Armand Thousand, Marcinek M, Zalewska A, Żukowska Chiliad, Wieczorek W (2010). "Detailed studies on the fillers modification and their influence on composite, poly(oxyethylene)-based polymeric electrolytes". Electrochimica Acta. 55 (iv): 1314–1322. doi:x.1016/j.electacta.2009.04.025. ISSN 0013-4686.
- ^ Syzdek J, Armand M, Gizowska M, Marcinek Grand, Sasim E, Szafran M, Wieczorek West (2009). "Ceramic-in-polymer versus polymer-in-ceramic polymeric electrolytes—A novel approach". Journal of Power Sources. 194 (1): 66–72. Bibcode:2009JPS...194...66S. doi:ten.1016/j.jpowsour.2009.01.070. ISSN 0378-7753.
- ^ Jiangshui Luo; Olaf Conrad; Ivo F. J. Vankelecom (2013). "Imidazolium methanesulfonate every bit a loftier temperature proton conductor". Periodical of Materials Chemistry A. 1 (six): 2238–2247. doi:ten.1039/C2TA00713D. S2CID 96622511.
External links [edit]
-
 Media related to Electrolytes at Wikimedia Commons
Media related to Electrolytes at Wikimedia Commons - Friedman, Harold L. (1960). "Mayer's Ionic Solution Theory Applied to Electrolyte Mixtures". The Journal of Chemical Physics. 32 (4): 1134–1149. doi:ten.1063/1.1730863.
- Leaist, Derek K.; Lyons, Philip A. (1981). "Multicomponent improvidence of electrolytes with incomplete dissociation. Diffusion in a buffer solution". The Journal of Physical Chemical science. 85 (12): 1756–1762. doi:10.1021/j150612a033.
- Kaminsky, Manfred (1957). "Ion-solvent interaction and the viscosity of stiff-electrolyte solutions". Discussions of the Faraday Order. 24: 171. doi:10.1039/DF9572400171.
Which Statement Describes An Electrolyte,
Source: https://en.wikipedia.org/wiki/Electrolyte
Posted by: reynaindread.blogspot.com


0 Response to "Which Statement Describes An Electrolyte"
Post a Comment